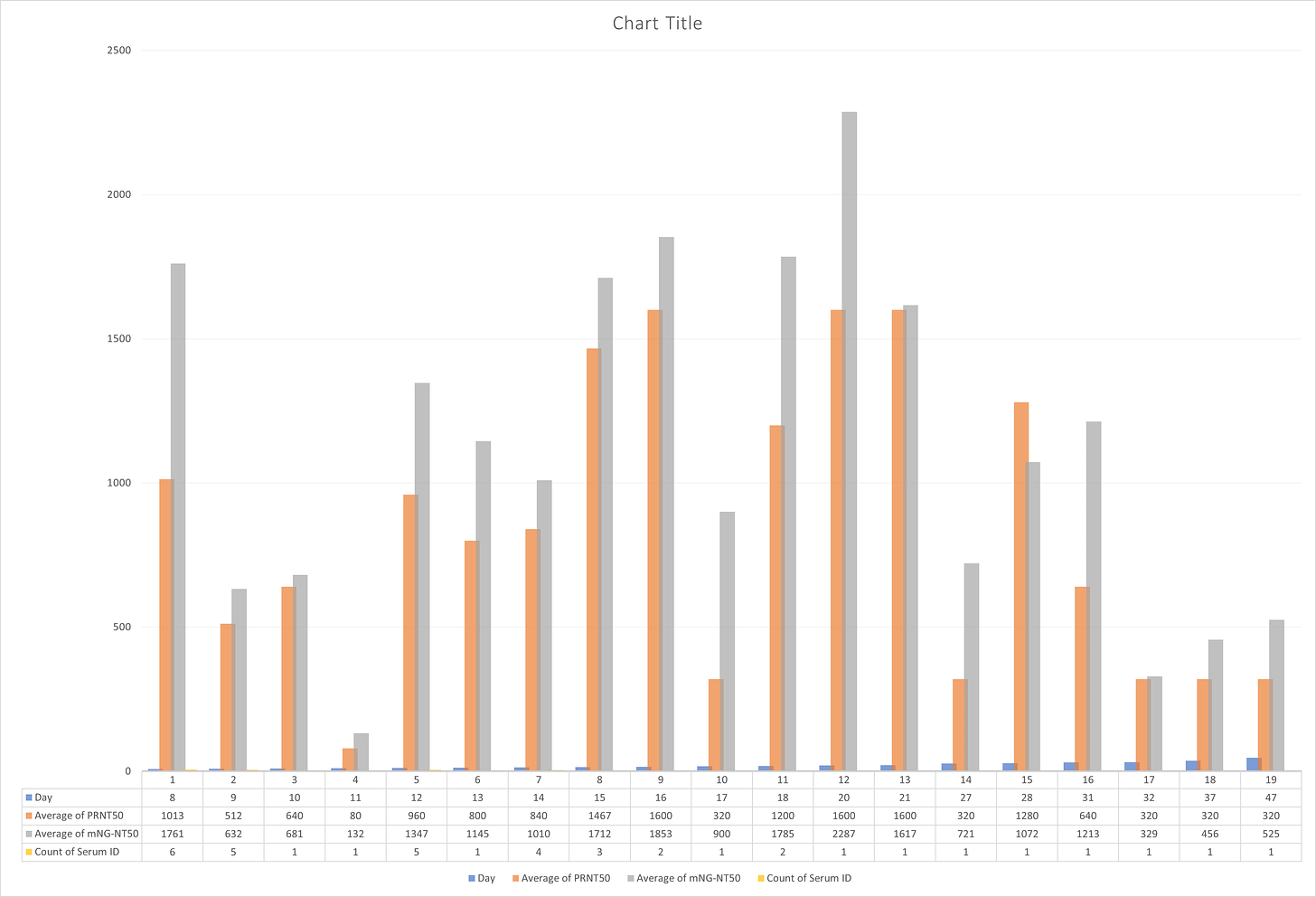How Pfizer & BioNTech Faked Their Pre-Clinical Studies
How Pfizer & BioNTech Faked Their Pre-Clinical Studies, Lied When They Claimed Their “Vaccine” Provides 8 Times Better Protection Than Natural Immunity, and How No One Said a Word About It
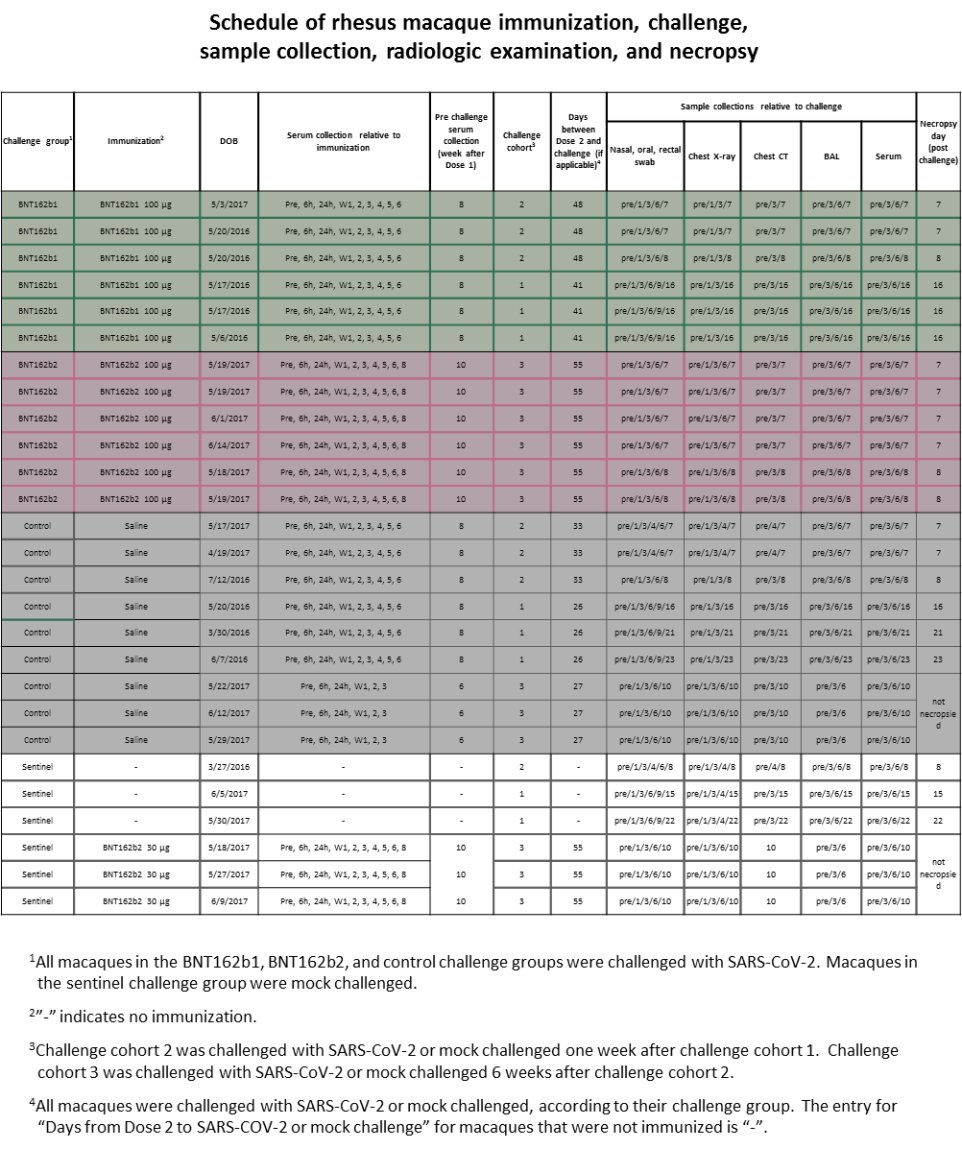
CHAPTER 1 - THE SCHEDULE
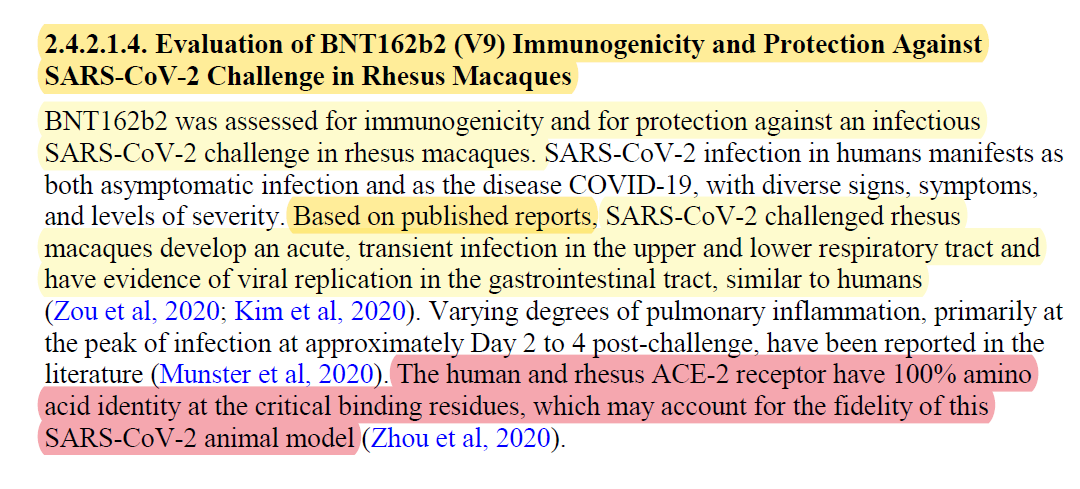
On the 1st of February 2021, Nature magazine published an article entitled "BNT162b vaccines protect rhesus macaques from SARS-CoV-2". It was the only article Pfizer/BioNTech has published in academic literature about their animals pre-clinical experiments.
The information that was published in this paper was provided to all the “health authorities” around the world and the claims in it, especially the claim that their product is 8 times more effective than natural immunity, has been weaponised by the criminal syndication to create a false narrative that has been at the heart of the totalitarian state we been living under since 2020, as well as playing a CRITICAL role in Pfizer/BioNTech receiving the Emergency Use Authorization.
I've covered the usage of animal which were unable to get sick by SARS-CoV-2 in my previous article, The Rats, and the meaning it has about the safety of the “vaccine”. If you haven’t read it yet, it is highly recommended.
Here is the 1st quote from Pfizer/BioNTech "Module 2.4. Nonclinical Overview", which covered the monkey studies. If you read The Rats, you know that the claim highlighted in red is a lie, and that everyone who worked in this field knew it is one.
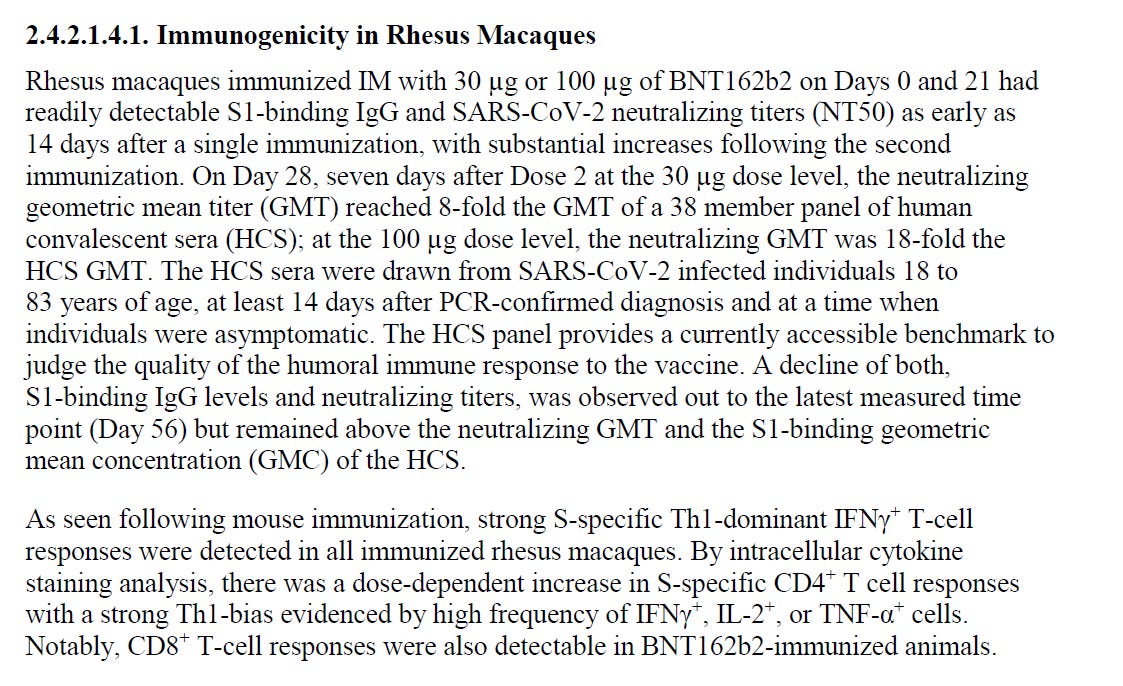
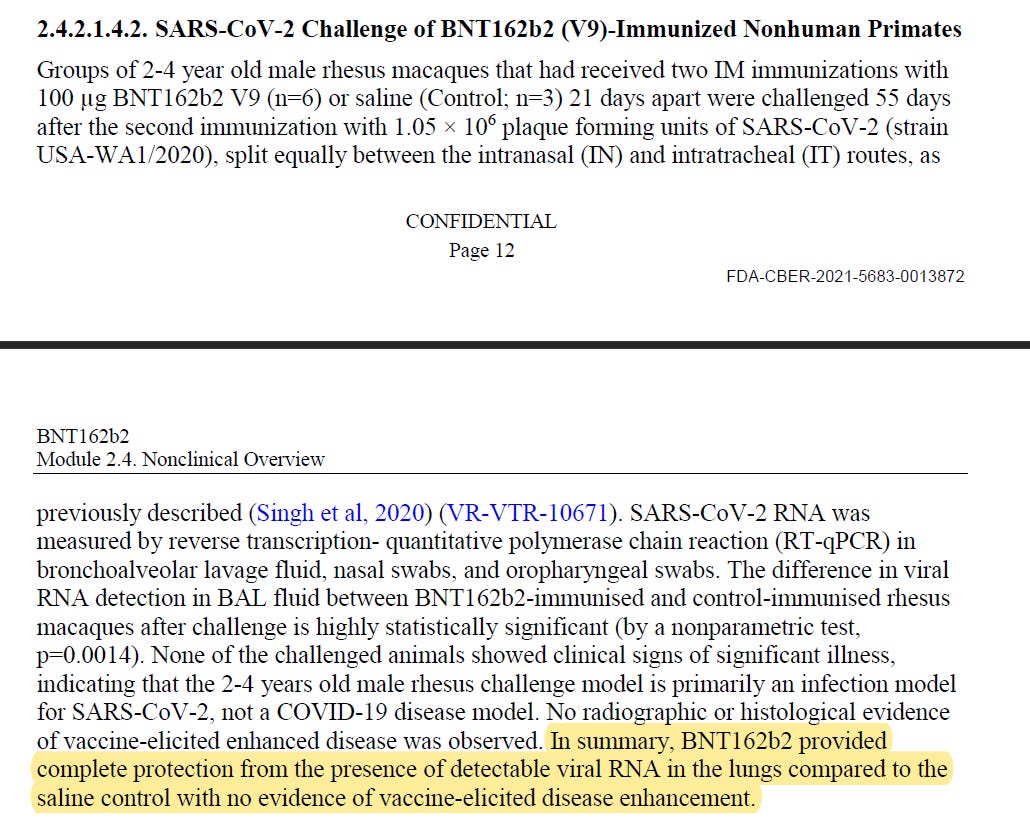
And here are more quotes from Pfizer/BioNTech "Module 2.4. Nonclinical Overview" about the monkeys studies.
Looks impressive, doesn't it? Of course it does.
Sadly, as you are about to find out, it's all fake.
Let's go back to the research paper that was published in Nature magazine, which was first disclosed by Pfizer/BioNTech as a on the September 8th, 2020. (more on that on Chapter 2). Notice the date - this means health regulators had it prior to their EUA approval.
FAKE MONKEYS = FAKE RESEARCH
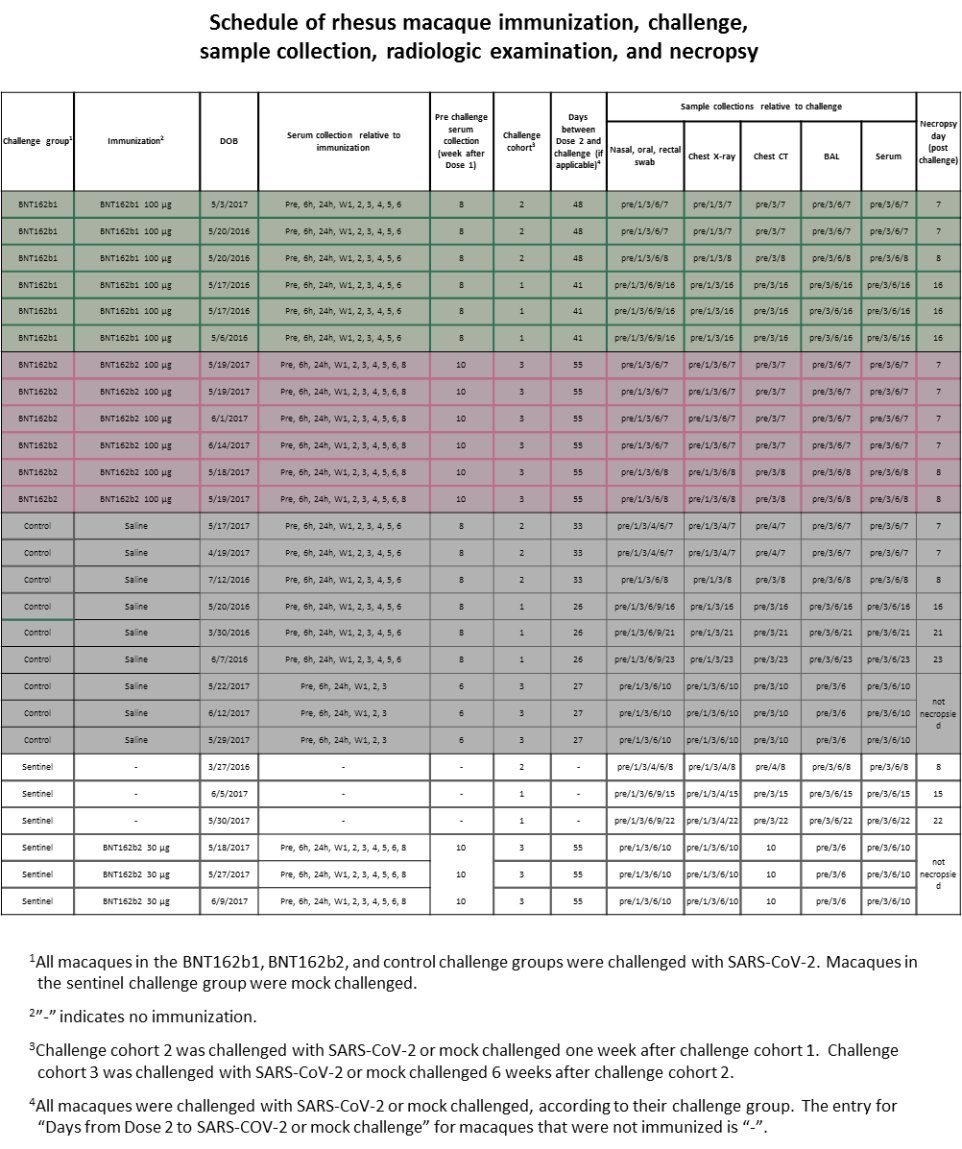
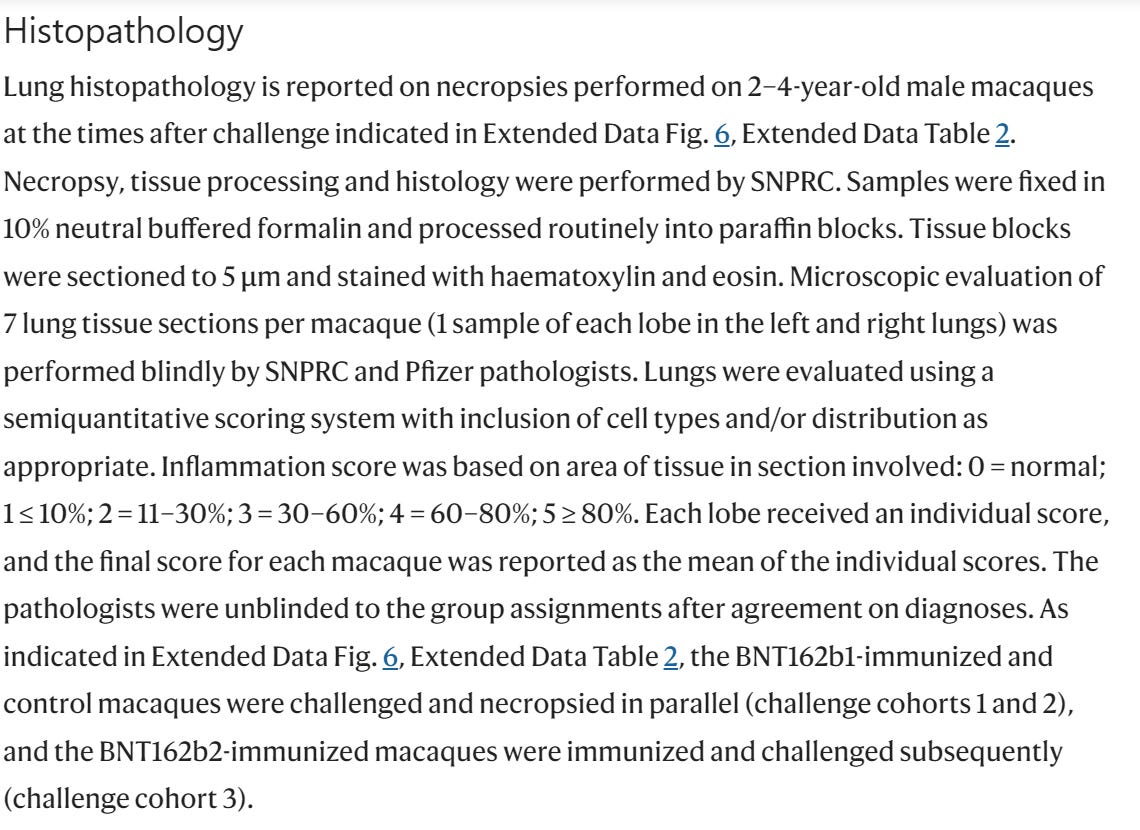
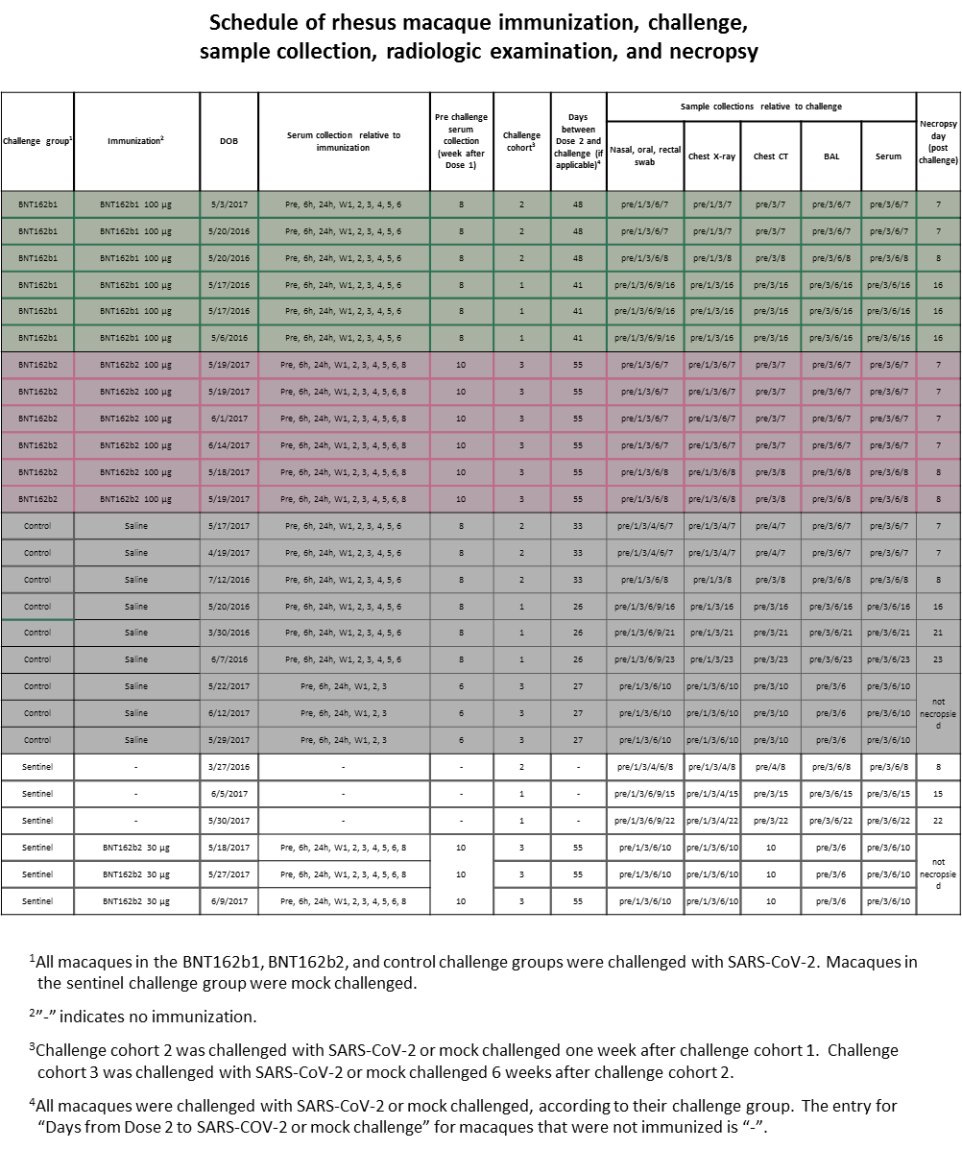
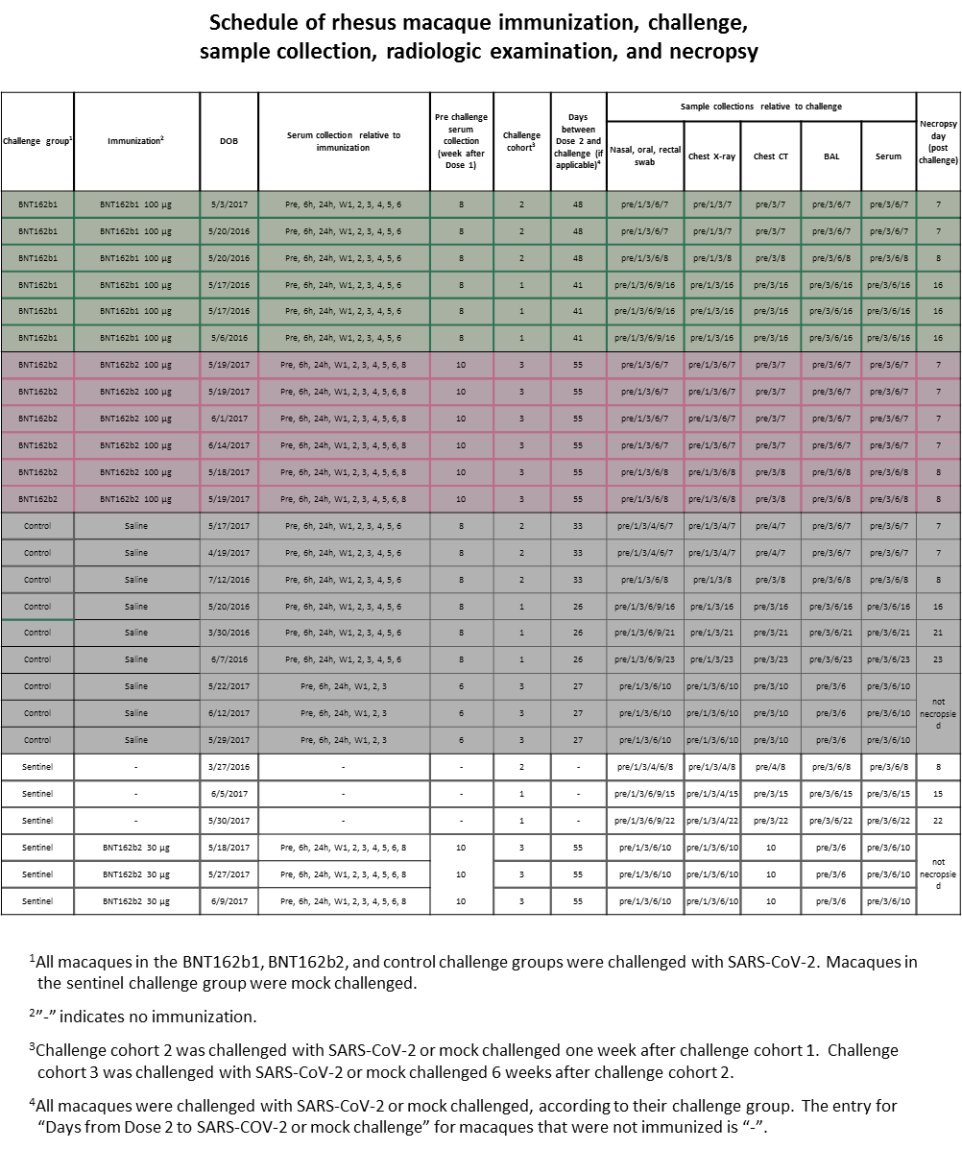
There are A LOT of things that should have alerted the regulators (and those who performed the peer-review for Nature magazine), but in this chapter we will focus on one of them, the schedule of the study according to Pfizer/BioNTech, and related results. According to Pfizer/BioNTech "Extended Data Figure 6." there were ZERO monkeys who received BNT162b1 30μg.
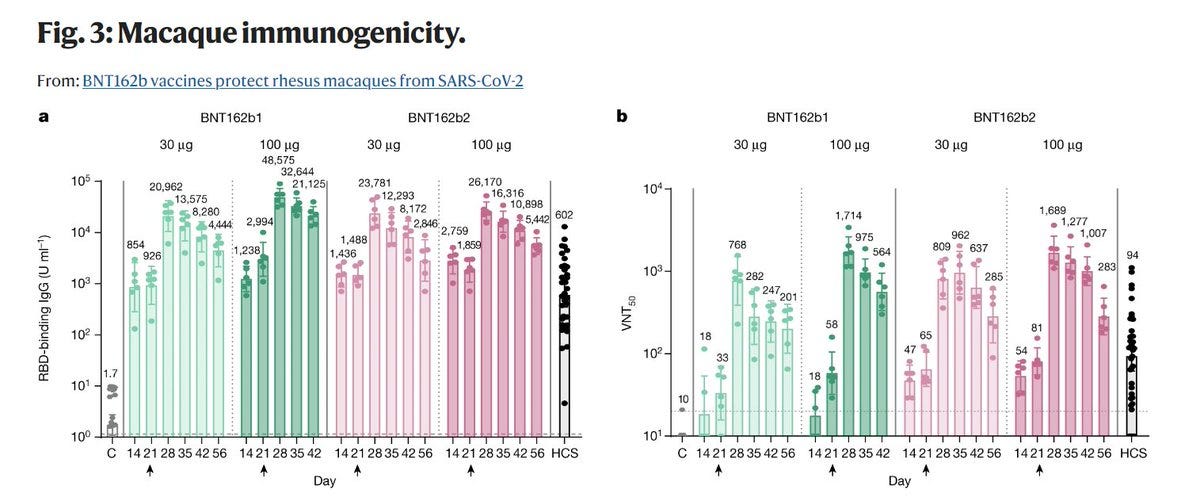
AND YET IN "Fig. 3: Macaque immunogenicity" (see below) THERE ARE RESULTS OF 6 MONKEYS WHO "RECEIVED" BNT162b1 30μg. Who were these monkeys??? Where did they came from?
The information above alone should have resulted an investigation of fraud. The fact that Nature did not do anything makes them complicit in an act of fraud.
Let’s continue.
According to Pfizer/BioNTech "Extended Data Figure 6." there were 3 monkeys who received BNT162b2 30μg, as part of a "sentinel group".
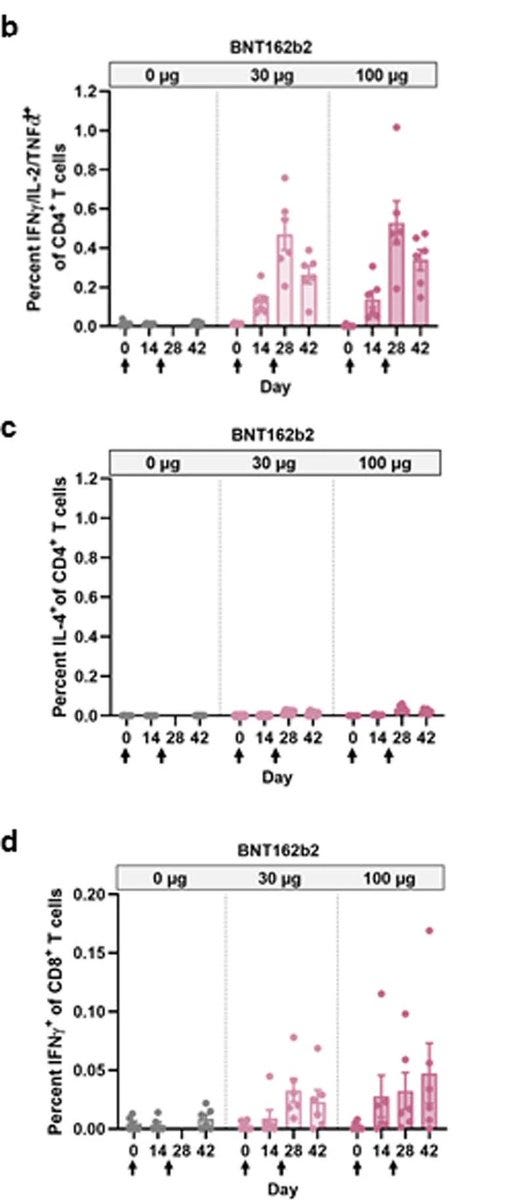
And yet in "Extended Data Fig. 5: Macaque CD4+ and CD8+ T cell response" there are results of 6 monkeys with BNT162b2 30μg.
See for yourself:
AGAIN, The information above alone should have resulted an investigation of fraud. The fact that Nature did not do anything makes them complicit in an act of fraud.
Let’s continue.
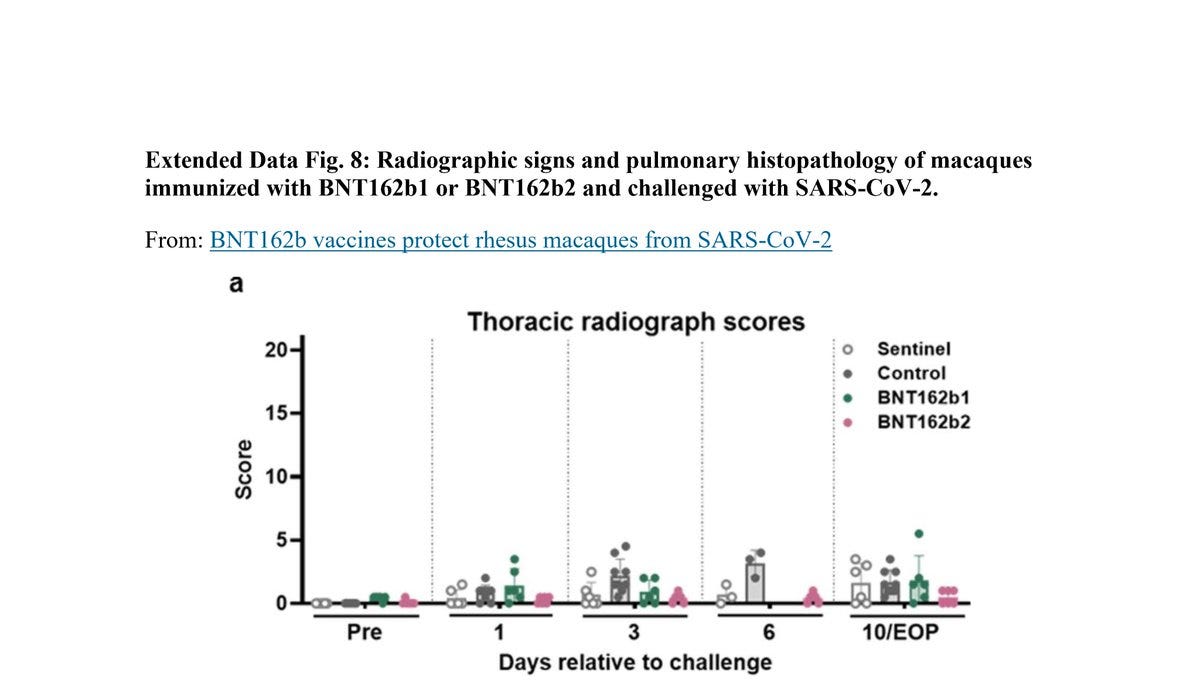
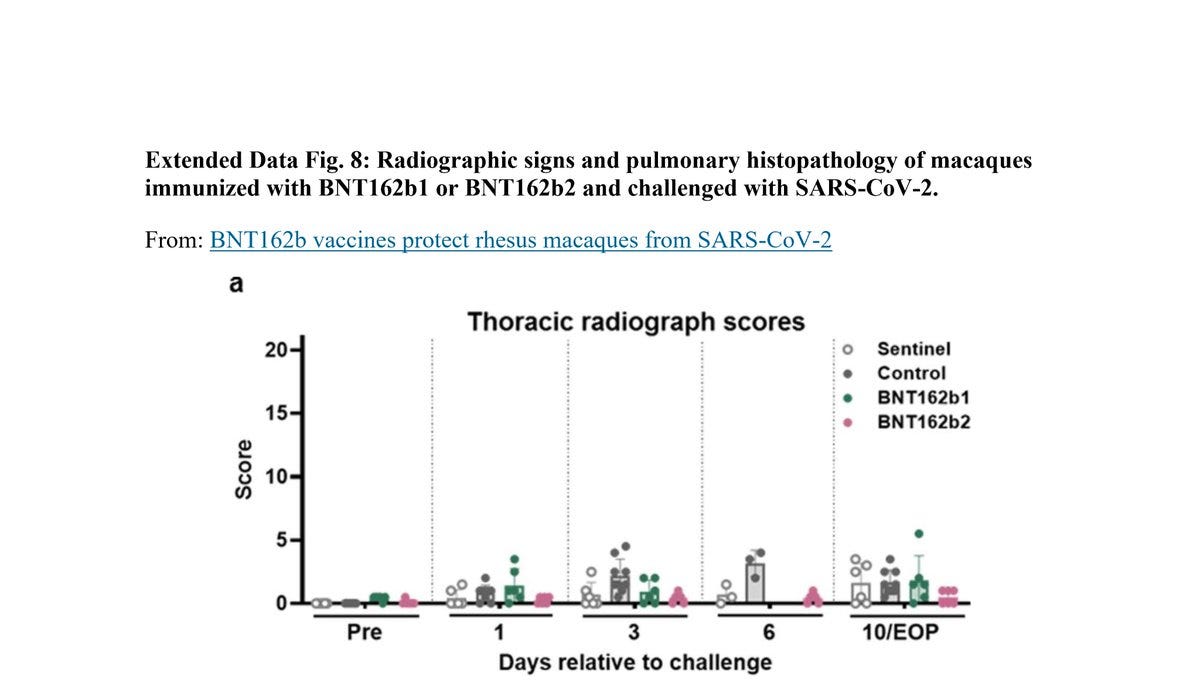
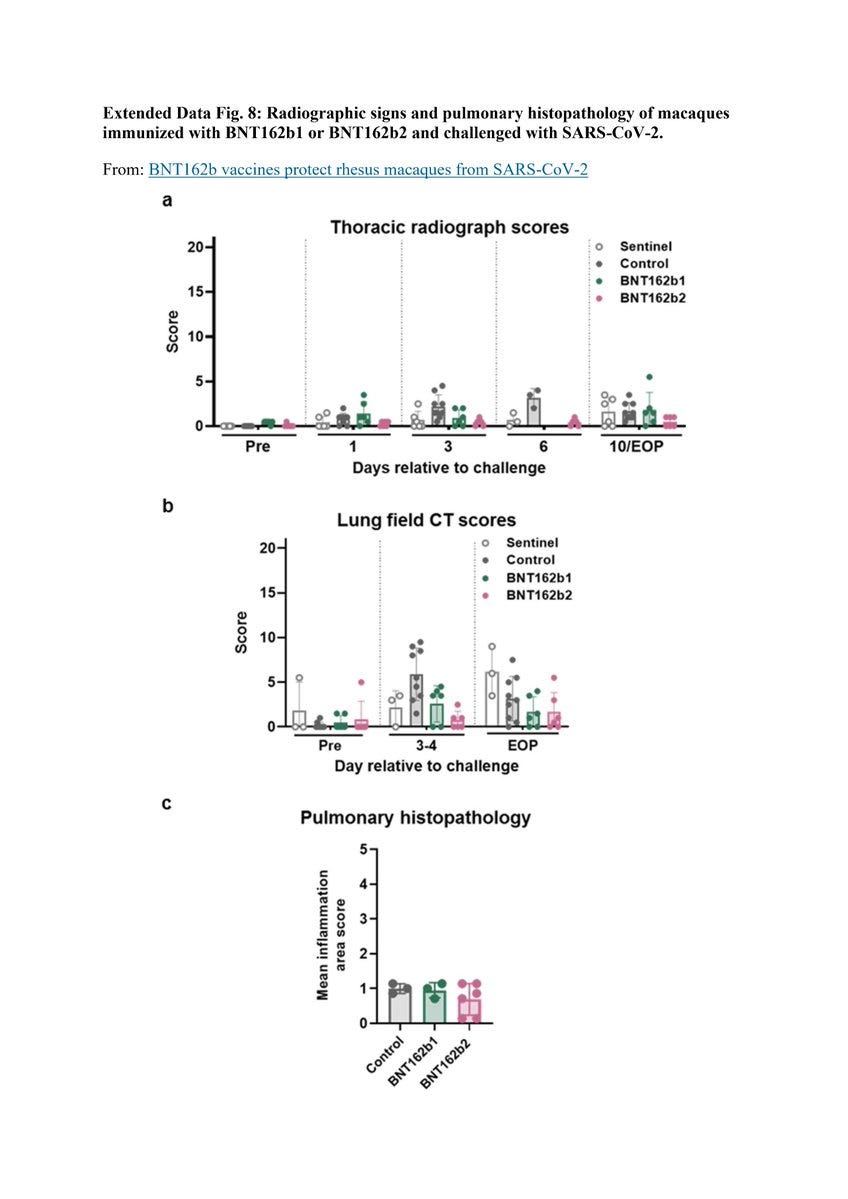
Here is "Extended Data Fig. 8". Notice how day 6 in "Thoracic Radiograph Scores":
Chest X-Ray is missing scores for the following monkeys:
-
3 "Sentinel"
-
6 "control"
-
6 "BNT162b1"
Why did Pfizer/BioNTech decided not to test them on day 6? Why choose a specific day? Why didn’t anybody ask?
Let’s continue
More on "Extended Data Fig. 8" X-ray:
Pfizer/BioNTech used a score of 0 to 3 for 7 lung regions, and the "Sentinel" group were not challenged with SARS-CoV-2:
Could it be that the 3 monkeys in that group that has higher score in 10/EOP are the BNT162b2 30μg? How can anyone tell? Why create a sentinel group that allows Pfizer/BioNTech not to present their data?
Let’s continue
"Extended Data Fig. 8" also covered chest CT scores:
How come the "Sentinel" group only shows scores to 3 monkeys, not 6? How come Pulmonary Histopathology is missing for the sentinel group? Was Pfizer/BioNTech trying to hide something?
Let’s continue.
challenging/Rechallenging
According to Pfizer/BioNTech, "The three control macaques in challenge cohort 3 and three sentinel macaques were not necropsied to allow their subsequent rechallenge (control) or challenge (sentinel)".
However, according to Pfizer/BioNTech cohort 3 occurred after cohort 1 & 2, so what challenge and rechallenge are we talking about here? Rechallenge to what? Challenge to what? What's going on?
More questions:
-
Why did Pfizer/BioNTech created a sentinel group?
-
Why was there no autopsy for the 3 BNT162b2 30μg monkeys?
-
WHO THE HELL REVIEWED THIS RESEARCH PRIOR TO EUA AND APPROVED IT?
-
WHO THE HELL APPROVED TO PUBLISH IT IN NATURE MAGAZINE?
Remember: The information above should have resulted an investigation of fraud. The fact that Nature did not do anything makes them complicit in an act of fraud.
CHAPTER 2 - THE PREPRINT
On the 9th of September 2020 Pfizer and BioNTech announced to the world in a PR entitled “Pfizer and BioNTech Announce Data from Preclinical Studies of mRNA-based Vaccine Candidate Against COVID-19” that their lead candidate "vaccine" formulation, called BNT162b2, has resulted in strong anti-viral effects against an infectious #SARSCoV2.
In the PR, Ugur Sahin, the BioNTech CEO has stated that “The data…include the characterization of our lead candidate BNT162b2, as well as key animal studies that were the basis for our clinical programs. THEY HAVE ENABLED US TO ADVANCE BNT162b2 INTO PHASE 3 EVALUATION”.
Pfizer’s senior VP & head of vaccine research & development, Kathrin U. Jansen, has stated that they (and BioNTech) were "encouraged by the data thus far and confident in our progress towards developing a safe and effective vaccine candidate to help address this current pandemic".
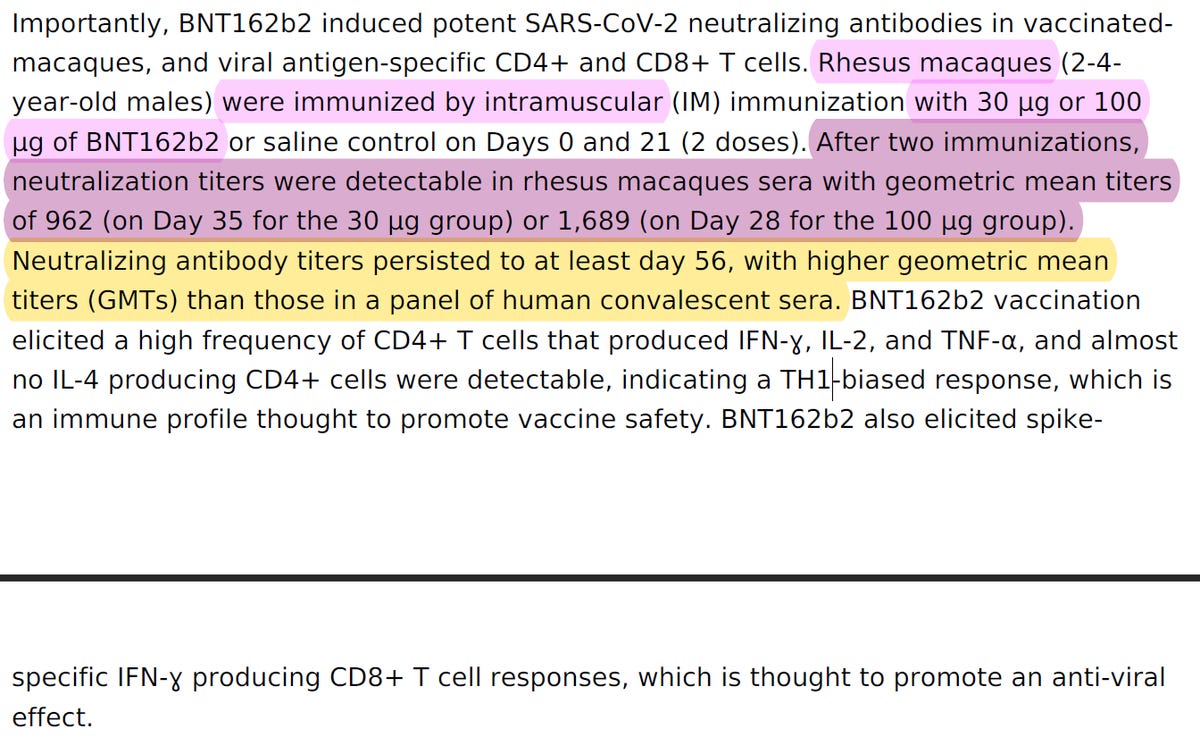
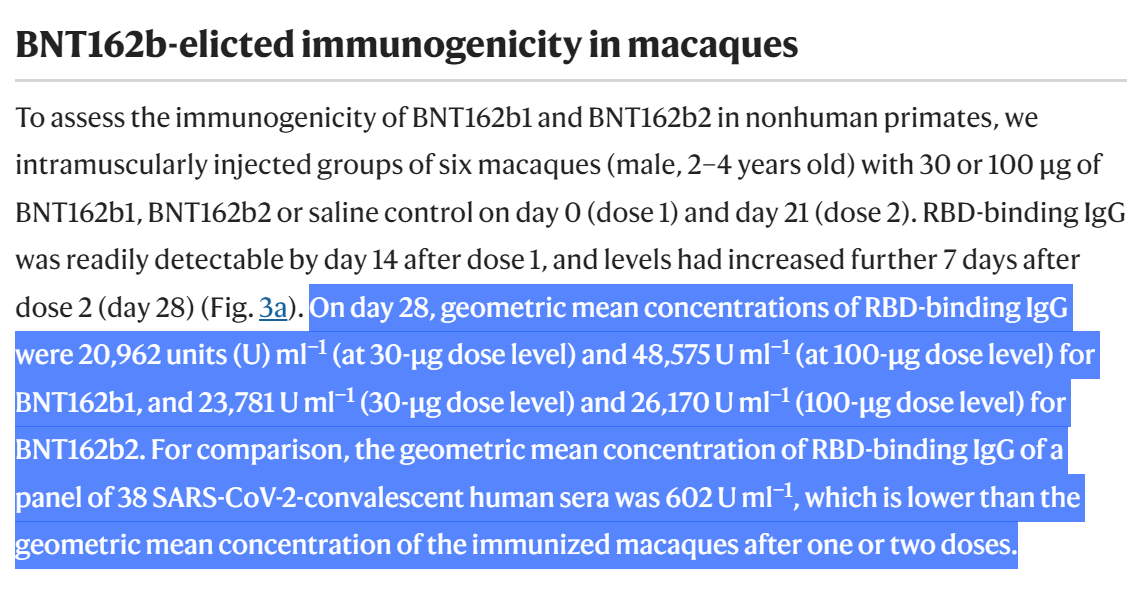
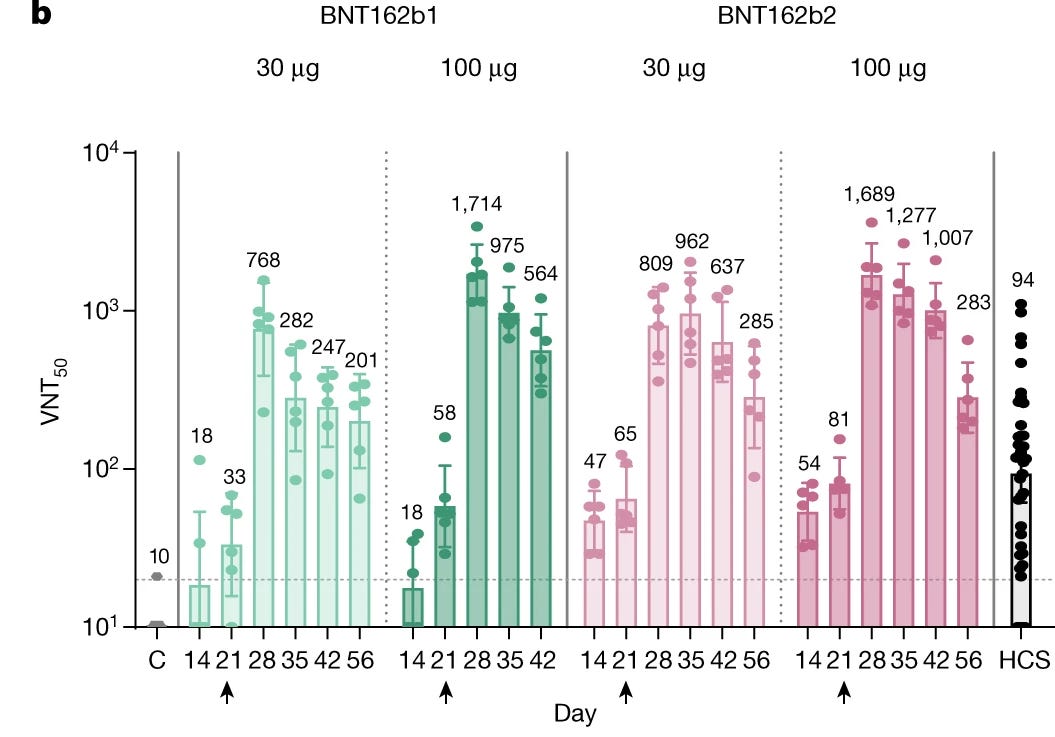
BioNTech/Pfizer has explained in the PR that "After two immunizations, neutralization titers were detectable in rhesus macaques sera with geometric mean titers of 962 (on Day 35 for the 30 µg group) or 1,689 (on Day 28 for the 100 µg group)."
In the PR, Pfizer (and BioNTech) provided a link to a preprint of their research, which was posted on September 8, 2020, and stated that it "is concurrently undergoing scientific peer-review for potential publication."
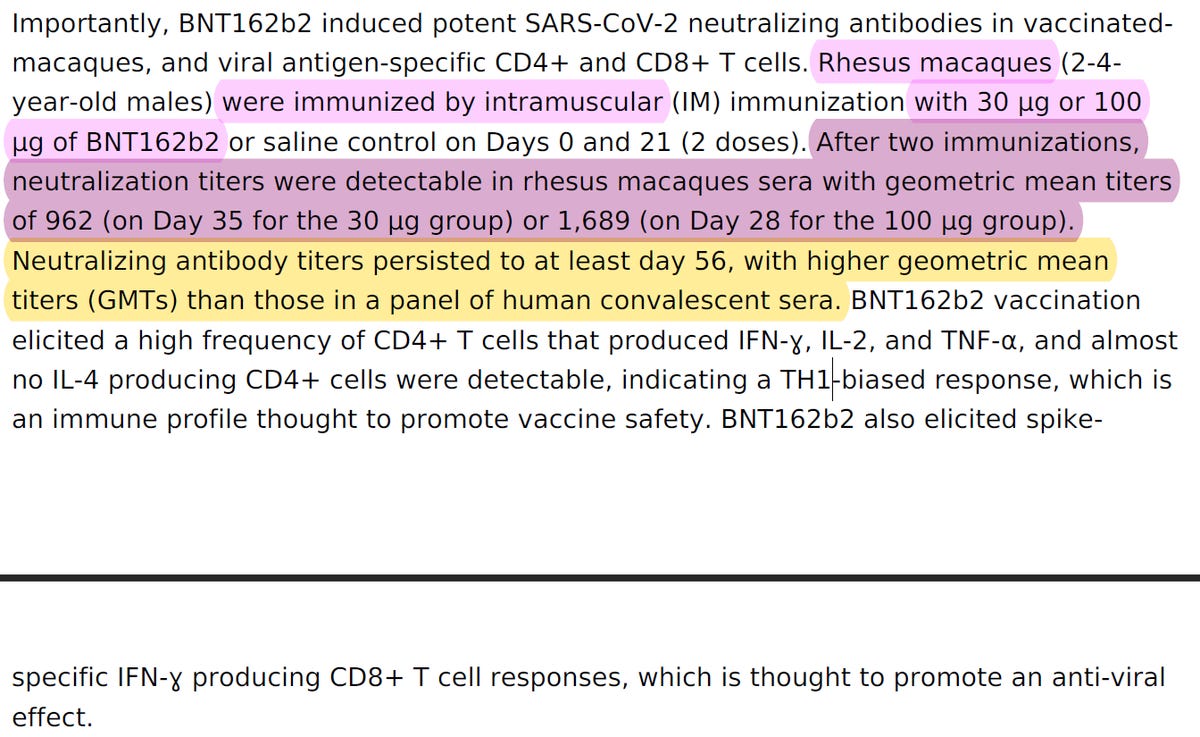
If you recall from chapter 1, in "Figure 3. Rhesus macaque immunogenicity", Pfizer & BioNTech has stated that: "Rhesus macaques (n=6 per group) were immunised on Days 0 and 21 with 30μg or 100μg BNT162b2 or buffer".
These results WERE 100% FAKE. Allow me to explain why…
A week before that, on the 1st of September 2020, BioNTech & Pfizer has submitted ANOTHER paper, this time to Nature magazine, which was later published it on the 1st of Feb 2021.
Take a look at the following chart, compare it to the preprint above, and notice difference between the two…
While in the preprint BioNTech & Pfizer has stated that On day 28 the GMCs of S1-binding IgG were 30,339 units (U)/mL (30 μg dose level), in paper they submitted to Nature they stated that the "geometric mean concentrations of RBD-binding IgG were 20,962 units (U) ml−1".
Wrong Measurements?
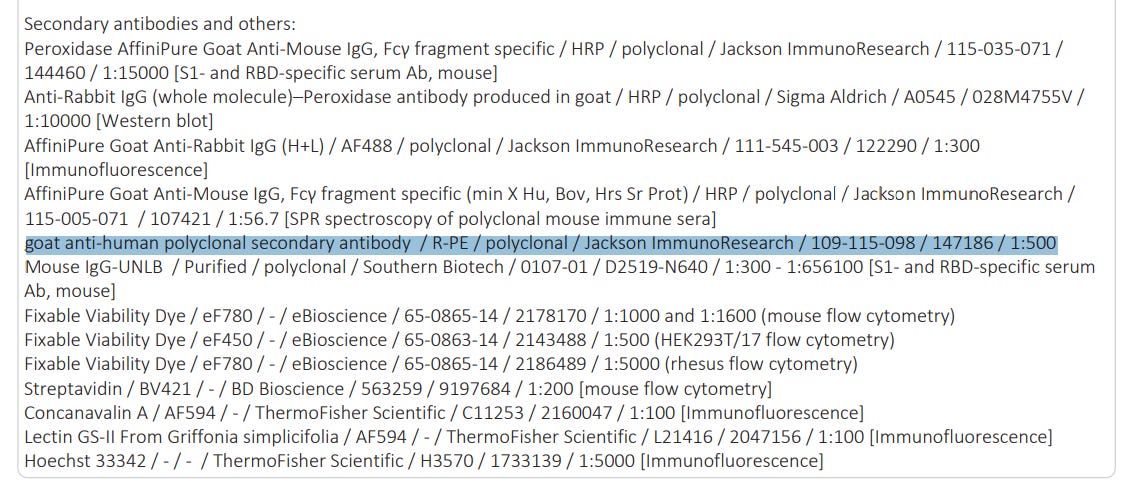
Let’s look at how BioNTech/Pfizer has measured the IgG response. Here is what they wrote in their publication, under “Analysis of S1- and RBD-specific serum IgG”:
Macaques and humans
Recombinant SARS-CoV-2 S1 containing a C-terminal Avitag (Acro Biosystems) was bound to streptavidin-coated Luminex microspheres. Bound macaque or human anti-S1 antibodies present in the serum were detected with a fluorescently labelled goat anti-human polyclonal secondary antibody (Jackson ImmunoResearch). Data were captured as median fluorescent intensities using a Bioplex200 system (Bio-Rad) and converted to U ml−1 antibody concentrations using a reference standard consisting of 5 pooled SARS-CoV-2-convalescent human serum samples (obtained >14 days after PCR diagnosis, from the panel described in ‘Panel of SARS-CoV-2-convalescent human sera’), diluted in antibody-depleted human serum with arbitrary assigned concentrations of 100 U ml−1 and accounting for the serum dilution factor.
According to BioNTech/Pfizer’s own submission, this is what they used:
Which is this product:
So this test works when the antibody reacts with the Fc portion of human IgG heavy chain but not with the Fab portion of human IgG.
What’s the difference?
The Fc portion of human IgG is the fragment crystallizable region, which is located at the tail end of the immunoglobulin molecule. It is responsible for the immunological functions of IgG, such as complement activation and antibody-dependent cellular cytotoxicity. The Fab portion of human IgG, on the other hand, is the fragment antigen-binding region, which is located at the head of the immunoglobulin molecule. It is responsible for the specific recognition and binding of antigens. In summary, the Fc portion is responsible for the immunological functions and the Fab portion is responsible for the specific antigen binding.
The Fab portion of human IgG is responsible for the specific recognition and binding of antigens, including viral antigens. When a virus infects a host cell, it displays viral antigens on its surface. These antigens are recognized by B cells that have antibodies specific to the viral antigen on their surface. Once the B cell recognizes the viral antigen, it will proliferate and differentiate into plasma cells that produce more antibodies. These antibodies, also known as immunoglobulins, are secreted into the bloodstream and can bind to the viral antigen on the surface of the virus, neutralizing it and preventing it from infecting more host cells.
The Fc portion of human IgG does not directly neutralize the virus, but it does play a role in the immune response. The Fc portion of IgG can bind to Fc receptors on the surface of immune cells, such as macrophages and natural killer cells. This binding leads to the activation of these cells, which can then help to clear the virus from the body.
It is the Fab portion of human IgG that recognizes and binds to the viral antigen, neutralizing the virus while the Fc portion plays a role in activating the immune cells to clear the virus from the body.
What’s the problem? The Fc portion of IgG is responsible for the immunological functions of the antibody. This sequence of amino acids that make up the Fc portion of IgG is very similar in different species. However, there are some variations in the amino acid sequence between the Fc portion of human IgG and the Fc portion of monkey IgG.
These variations in amino acid sequences can result in differences in the way that the Fc portion of IgG from different species interacts with Fc receptors, which are proteins found on the surface of immune cells. These variations can also affect the way that the IgG molecules from different species interact with other molecules in the immune system, such as complement proteins.
In “IgG Fc variant cross-reactivity between human and rhesus macaque FcγRs”, publish in 2017, the authors have written that “The resulting data indicate that amino acid variation present in rhesus FcγRs can result in disrupted, matched, or even increased affinity of IgG Fc variants compared with human FcγR orthologs. These observations emphasize the importance of evaluating species cross-reactivity and developing an understanding of the potential limitations or suitability of representative in vitro and in vivo models before human clinical studies when either efficacy or toxicity may be associated with FcγR engagement.”
You can read more about it in “Mind the Gap: How Interspecies Variability in IgG and Its Receptors May Complicate Comparisons of Human and Non-human Primate Effector Function”
So, to summarize this part:
-
This was NOT a specific test to identify specific SARS-CoV-2 S-1 or RDB antibodies, but a general test to identify a specific part of the FC portion of a human IgG.
-
Due to the genetic differences between human IgG Fc and the macaque, comparing results is simply wrong.
So when you read this… realise it is like comparing apples and oranges:
Also, in the paper BioNTech/Pfizer stated that "groups of six male, 2-4 year old rhesus macaques were immunised IM with 30 or 100 μg of BNT162b2", which I've proved in chapter 1 was a false statement,. They only had 3 monkeys who received 30 μg of BNT162b2.
If BioNTech/Pfizer had no problem publishing pre-clinical results that contradicted a paper they submitted for publication only a week earlier, and if their papers were so full of inaccuracies, how much can trust their phase 1/2/3 clinical trials results or anything they ever published?
Final, and last point for this chapter:
The REAL submission date of the article to Nature magazine was on the 1st of September 2020, as you can clearly see it got in the document itself, it was submitted via “Accelerated Review” process.
Why? See the next chapter.
CHAPTER 3 - THE NEAUTRALIZING DEFECT
QUICK RECAP:
When BioNTech/Pfizer submitted Emergency Use Authorization (EUA) of their "vaccine", they presented before the regulators a set of pre-clinical studies. One of them, VR-VTR-10671, was performed in male rhesus macaques. This was such an important study that BioNTech/Pfizer not only submitted it to Nature magazine, and got it published, but also used it to claim that their selected product, entitled BNT162b2, was effective in neutralizing SARS-CoV-2. BioNTech/Pfizer claimed that after two shots the level of neutralizing antibodies (which stops the virus from entering healthy cells) was 8 TIMES HIGHER THAN NATURAL IMMUNITY. They claimed they proved it via what is known as virus neutralization test, or VNT.
Normally, for BioNTech/Pfizer to prove such claim they would take the virus, cultivate it, add it to healthy cells, add a serum that contains the antibodies, and see after a few days if the serum managed to stop the infection. But this was SARS-CoV-2, which was considered to be a deadly pathogen. Working with SARS-CoV-2 required a biosafety-level 3 laboratory (BSL-3 lab). When BioNTech/Pfizer started their testing they didn't had access to such lab, so at first they had to come up with an alternatives.
For the mice experiments BioNTech/Pfizer used a vesicular stomatitis virus (VSV), which they genetically engineered it to have the coronavirus spike protein and a fluorescent green, glowing enzyme, which they used for their virus neutralization test (VNT).
With the monkeys study, BioNTech/Pfizer has chosen another method. Instead of using the VSV pseudovirus like they did with the mice, they decided to go with a method that was developed by a group of researchers in the University of Texas Medical Branch (UTMB). The method they used was later published in Nature magazine as "A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation". Two of the paper's authors joined BioNTech/Pfizer, became co-authors of the paper, and performed the measurements which were presented in the paper .
Glowing Success
Let's look how it was done:
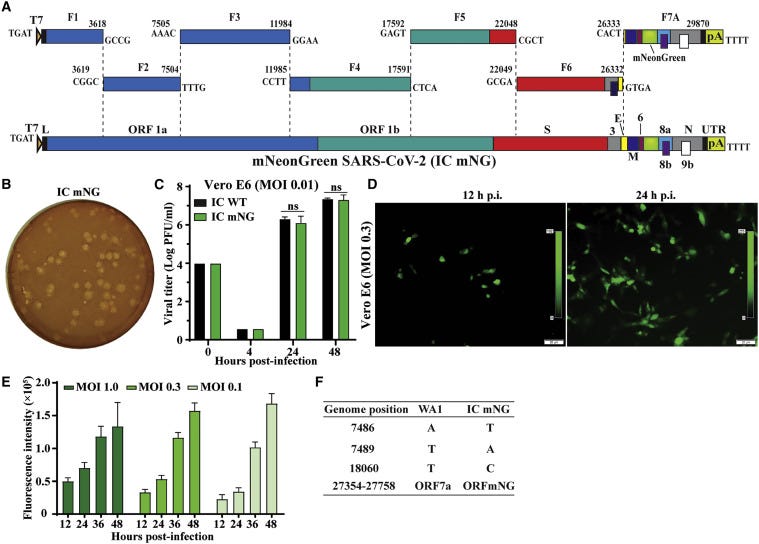
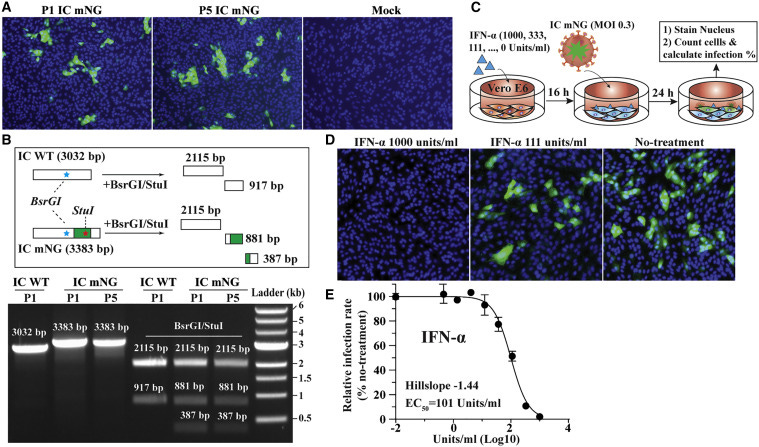
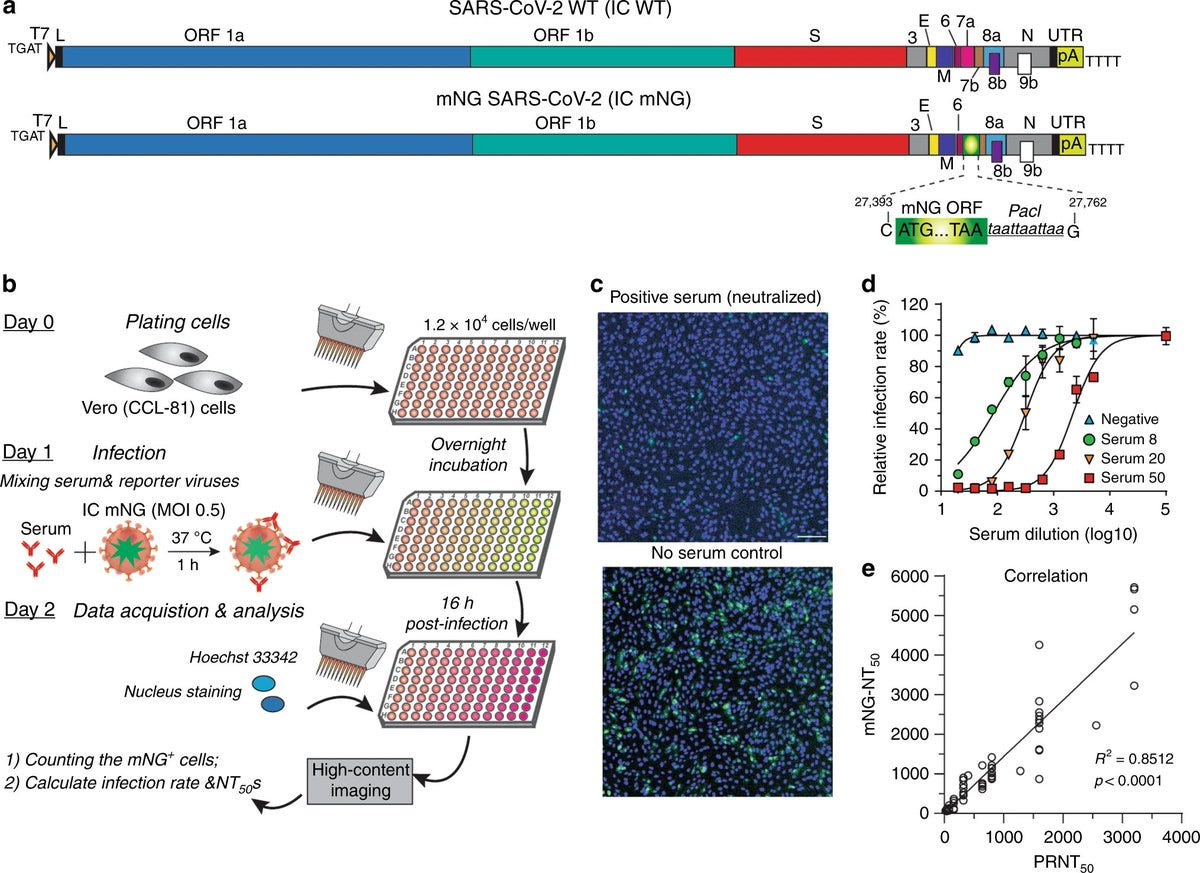
The researchers took the genetic code of the virus, and replace a segment of it (ORF7) with what is called a reporter mNG (mNeonGreen) gene, that allows them to observe & measure it. They used a method that was previously published in this paper. They researchers stated that "conventionally, neutralizing antibodies are measured by plaque reduction neutralization test (PRNT)…PRNT remains the gold standard for serological testing and determining immune protection".
The researchers came up with the new method for LARGE SCALE testing in a biosafety-level 3 laboratory. As they claimed in their paper, "due to its low throughput, PRNT is not practical for large scale serodiagnosis and vaccine evaluation".
VNT
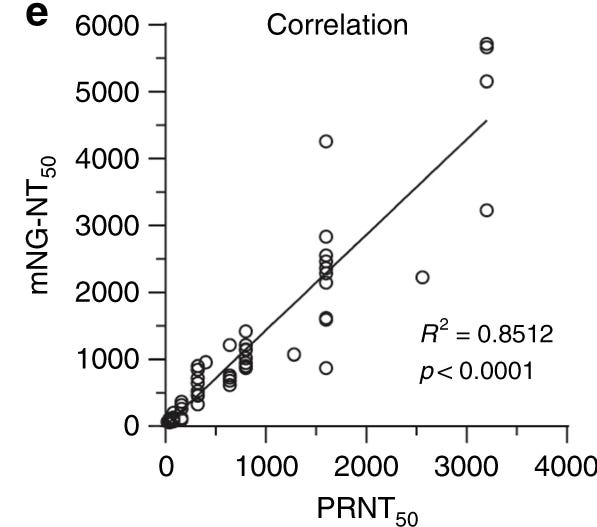
The values that are used to state the effectiveness of the antibodies are measured as 50% virus-neutralization titres (VNT). The new method measured neutralized 50% of fluorescent cells (NT50), while the gold standard PRNT measurement are expressed in PRNT50, and they didn't really match.
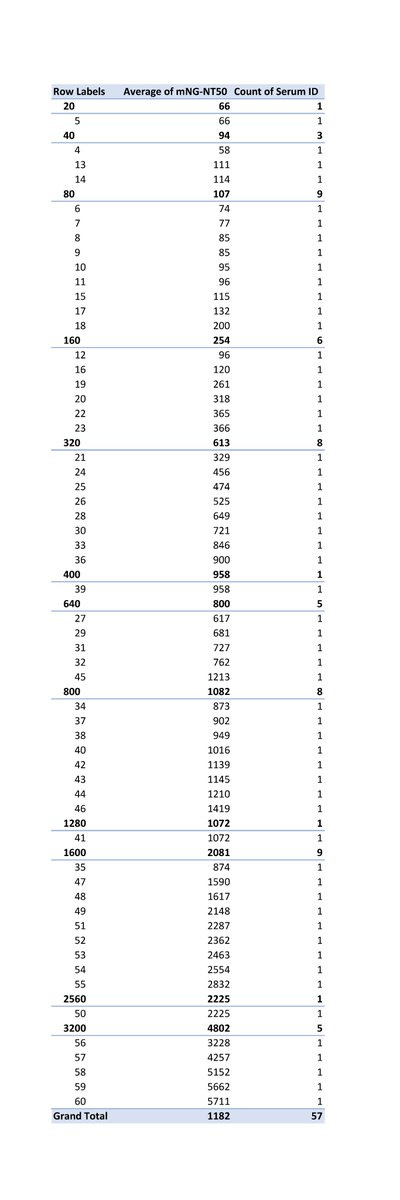
The researchers has used 60 COVID-19 serum specimens from RT-PCR-confirmed patients. 57 were valid. As you can see, in comparison to PRNT50, there was a big fluctuation in NT50 values, but there was a correlation efficiency R2 of 0.85.
Nerd Data Zone
OK, since average is not the best method of comparison, let’s do an analysis by day, based on the information we have about the original research sample data.
First, let’s clean up the data, and leave only samples information that we have information on the day, the level of PRTN50 and NT50. In case a value of PRTN50 is less than the threshold, let’s assign it a value of 0.
This is the data we get, sorted by days.
PRNT50 NT50 Day Serum ID
80 95 1 10
80 115 1 15
0 35 1 1
80 85 4 8
0 50 4 3
20 66 5 5
40 58 5 4
80 85 5 9
160 318 5 20
0 38 5 2
40 111 6 13
40 114 6 14
80 74 6 6
80 96 6 11
80 77 8 7
80 200 8 18
320 846 8 33
800 1419 8 46
1600 2362 8 52
3200 5662 8 59
160 120 9 16
320 649 9 28
640 727 9 31
640 762 9 32
800 902 9 37
640 681 10 29
80 132 11 17
160 366 12 23
640 617 12 27
800 1139 12 42
1600 2148 12 49
1600 2463 12 53
800 1145 13 43
160 365 14 22
800 873 14 34
800 1210 14 44
1600 1590 14 47
400 958 15 39
800 949 15 38
3200 3228 15 56
1600 874 16 35
1600 2832 16 55
320 900 17 36
800 1016 18 40
1600 2554 18 54
1600 2287 20 51
1600 1617 21 48
320 721 27 30
1280 1072 28 41
640 1213 31 45
320 329 32 21
320 456 37 24
320 525 47 26
And here are the averages, per day (APRNT50 = average PRNT; ANT = Average NT50):
DAY APRNT50 ANT50
1 53 82
4 40 68
5 60 113
6 60 99
8 1013 1761
9 512 632
10 640 681
11 80 132
12 960 1347
13 800 1145
14 840 1010
15 1467 1712
16 1600 1853
17 320 900
18 1200 1785
20 1600 2287
21 1600 1617
27 320 721
28 1280 1072
31 640 1213
32 320 329
37 320 456
47 320 525
Average of all:
: 676 931Now, let’s look at how it looks on a graph:
When we look at this sample range, the average of all PRTN50 is 676 and the average of mNG-NT50 is 931.
Let’s only look from day 8 forward (as days 1-7 seems to show little neutralizing activity), and let’s also include the sample size for each day:
When we look at this sample range (n=39), the average of all PRTN50 is 833 and the average of mNG-NT50 is 1115.
Back to BioNTech/Pfizer
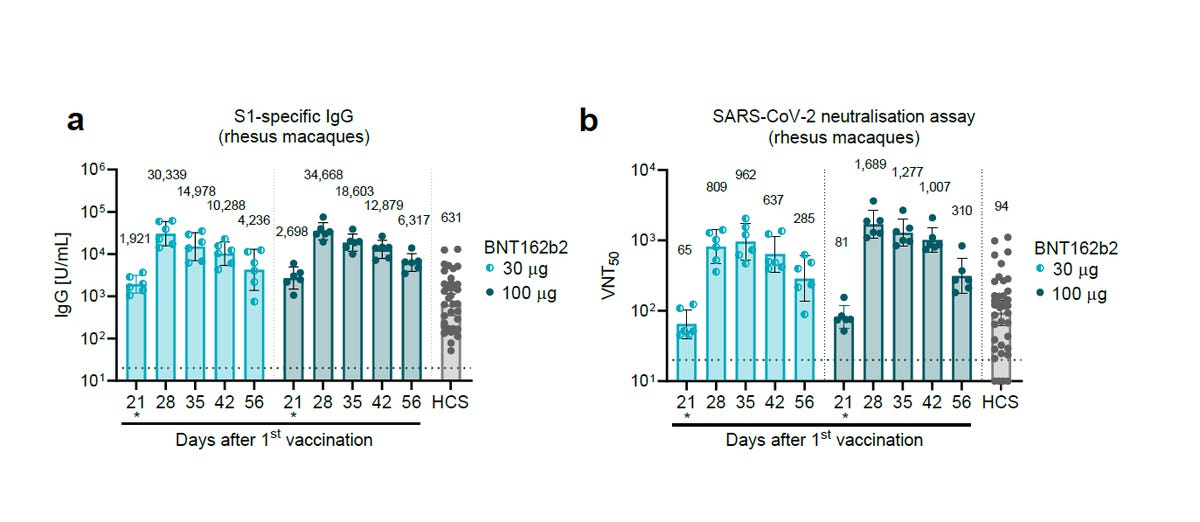
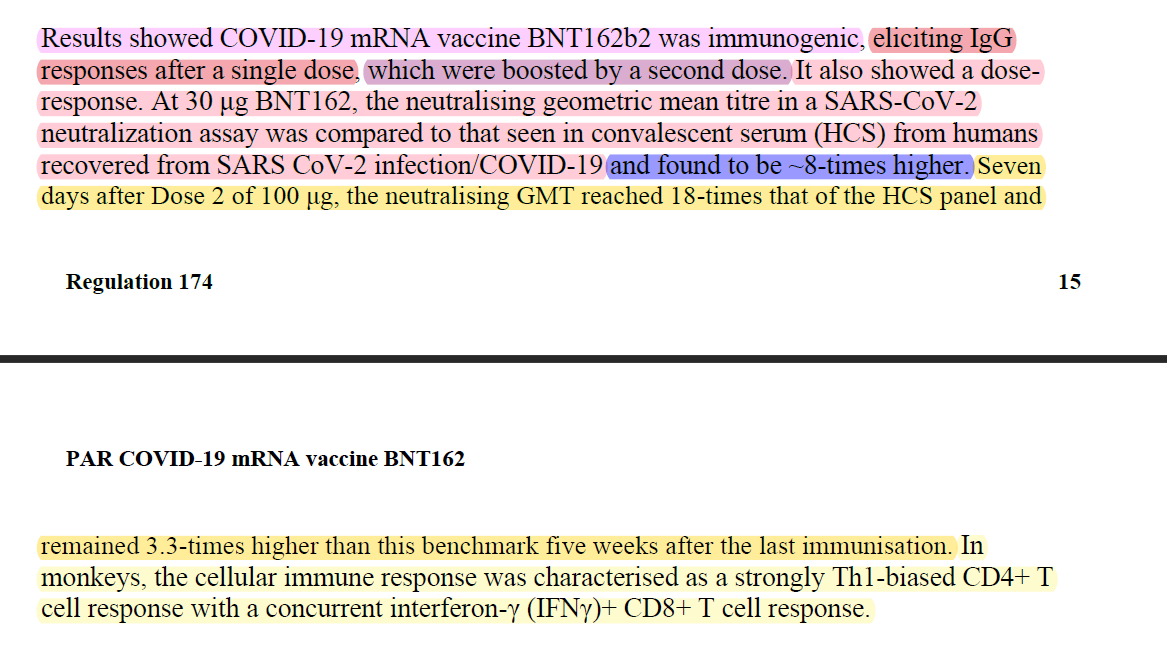
Here are the virus-neutralization titres (VNT) results from the study.
Notice a column called HCS? According to BioNTech/Pfizer, "Human convalescent sera (HCS) were obtained from (38) patients infected with SARS-CoV-2 at least 14 d after PCR-confirmed diagnosis and at a time when acute COVID-19 symptoms had resolved". BioNTech/Pfizer also stated that "The HCS panel is a benchmark for serology studies in this Article and previous publications".
One small point, from the paper: “Most serum donors had outpatient (35/38) or inpatient (1/38) COVID-19; 2 of 38 had asymptomatic SARS-CoV-2 infections. Sera were obtained from Sanguine Biosciences, the MT Group and Pfizer Occupational Health and Wellness”. Yes, Pfizer provided samples to what was supposed to be an objective measurement of the level of natural immunity (via the level of neutralizing antibodies), which they had all the incentives to show is not performing as well as their “vaccines”, and NO ONE said a word.
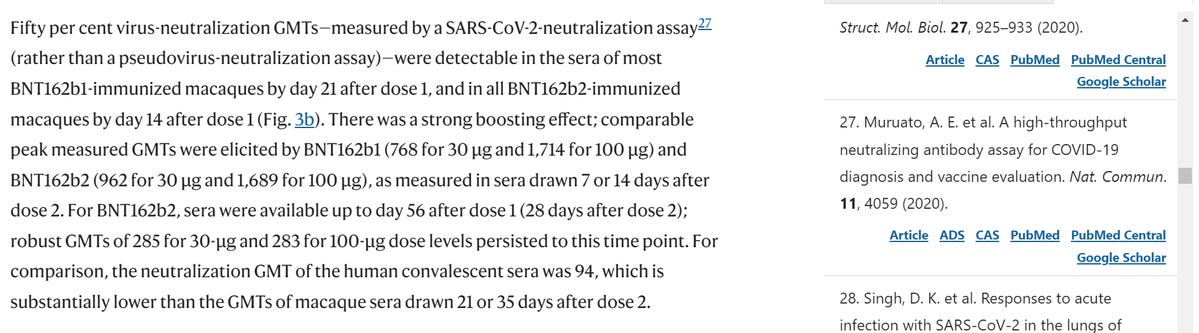
In BioNTech/Pfizer paper and in their regulatory submission, the VNT50 of BNT162b2 30μg on day 28 was 809, and the HCS VNT50 was 94. This meant, that based on this data, that on day 28 BNT162b2 30μg has elicited an 8 times higher level of neutralizing antibodies than a natural immunity.
What they FAILED to explain is HOW THE SAME 2 PEOPLE WHO CO-WROTE "A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation" ORIGINALLY FOUND THAT THE AVERAGE NT50 of the 39 SAMPLES OF SARS-CoV-2 POSITIVE PATIENTS FROM DAY 8 (as I explained above) WAS 1115!!!
It could be that the BioNTech/Pfizer HCS panel was flawed, but the 2 researchers who were involved in the original research and performed new measurements based on the method they developed would have noticed it and alert OR … the two researchers and Pfizer simply lied.
But why???
Follow the Money
Let’s look at the scientists that were involved in providing these measurements to Pfizer/BioNTech. Their names are Camila Fontes-Garfias and Pei-Yong Shi. Camila Fontes-Garfias was one of the two lead authors of the paper that described the VNT method used in the Pfizer/BioNTech method, and both of the authors were from the University of Texas Medical Branch, Galveston, TX, USA.
As stated in the paper, "C.F.-G. and P.-Y.S. performed and analysed VNT assays", and that “C.F.-G. and P.-Y.S. received compensation from Pfizer to perform neutralisation assays”
Show me the money !!!
In 2020, #PFIZER PAID $420,000 FOR "REPORTER SARSCOV2 VIRUS NEUTRALIZATION ASSAY" to the University of Texas Medical Branch (UTMB).
In July 2020: $100,000.
In August 2020: $200,000.
In Dec 2020: $120,000:
In 2021, #PFIZER PAID ANOTHER $1,319,000 FOR "REPORTER SARSCOV2 VIRUS NEUTRALIZATION ASSAY" to the University of Texas Medical Branch (UTMB).
-
832925047 - $100,000 (Jan 2021)
-
832924971 - $120,000 (Feb 2021)
-
832924961 - $120,000 (Feb 2021)
-
832925021 - $41,200 (Feb 2021)
-
832925037 - $300,000 (Mar 2021)
-
832501627 - $298,800 (Jun 2021)
-
832501635 - $339,000 (Oct 2021)
According to Open Payments, #Pfizer paid to the University of Texas Medical Branch (UTMB) for research $255,981 in 2019, $1,800,362 in 2020, and $3,703,434 in 2021.
Not only the amount of money that Pfizer had started to pay for research to the university of Texas Medical Branch in 2020 was SEVEN TIMES higher than it paid a year before, out of the total amounts of $ #Pfizer paid 23% of it in 2020 and 35% of it in 2021 went to "REPORTER SARSCOV2 VIRUS NEUTRALIZATION ASSAY", which is the work related the mRNA “vaccine”.
That is A LOT of funding for just one project, especially since this is a non-standard method!
It spells like "Research Payments", but it’s pronounced "bribery".
How can you trust scientists when such funding is funnel to them?
Oh, in case you wonder, The University of Texas Medical Branch has applied for a patent on this method of measuring…
WHO IS SHI?
One of the two scientists that performed for #Pfizer the virus neutralization assay analysis is Pei-Yong Shi, who is a CCP trained asset that is also an Honorary Professor at the Wuhan Institute of Virology.
In April 2022 the Epoch Times have revealed that the University of Texas Medical Branch (known as UTMB) Galveston National Laboratory has signed with the Wuhan lab a partnership agreement that stated labs must destroy ‘Secret Files’ upon request from the other lab.
Here Pei-Yong Shi connection to this lab:
Pei-Yong Shi is also an executive committee member of the Sealy Institute for Vaccine Sciences, a "WHO COLLABORATING CENTER" based in the University of Texas Medical Branch. He is also a past editor of multiple publications and current editorial board member of others such as Nature’s npj Vaccines.
Moving Out
I've mentioned there were two scientists who performed this analysis for #BioNTech/#Pfizer.
The 2nd person, who was one of the two lead authors of the original paper, Camila R. Fontes-Garfias, is no longer working for @utmbhealth but for…Pfizer since 03/21.
Questions to be answered:
-
Why would Pfizer/BioNTech prefer to go with a non-standard method? It's not as if they had to do mass testing - they only had 27 monkeys!
-
Why would regulators accept a method no one used before when there is a standard?
-
Who peer-reviewed this paper before its publication?
-
Where was the scientific community? Where were all the scientists who work in the field?
-
Where were the science and health journalists who cover this field?
-
Did anyone bothered reading the research which was submitted by BioNTech and Pfizer to the regulators? Did anyone checked if the data is valid? This dossier was submitted to ALL the regulators, all around the world. If no one saw what I saw, why would you trust any statements they make about the safety and efficiency of anything they authorise?
Copy & Paste
About the regulators, well, let’s look at MHRA, The UK's health regulator, which practically performed a copy & paste from #Pfizer/#BioNTech dossier when they requested the EUA. The health regulators don't work for us, they work for Big Pharma.
Cycling
(Due to popular demand lol)
As BioNTech/Prizer has described in their paper, “to detect and quantify SARS-CoV-2 in NHP, viral RNA was extracted from BAL fluid and from nasal, OP, and rectal swabs as previously described and tested by RT-qPCR as previously described (28)”
Reference 28 is entitled “Singh, D. K. et al. SARS-CoV-2 infection leads to acute infection with dynamic cellular and inflammatory flux in the lung that varies across nonhuman primate species. bioRxiv 2020.06.05.136481; 10.1101/2020.06.05.136481 (2020)”.
And here is a quote from the paper, describing how they perform the measurement:
“The SARS-CoV-2 RT-qPCR was performed using a CDC-developed 2019-nCoV_N1 assay with the TaqPath™ 1-Step RT-qPCR Master Mix, CG (ThermoFisher). The assays were performed on a QuantStudio 3 instrument (Applied Biosystems) with the following cycling parameters:
-
Hold stage 2 min at 25°C, 15 min at 50°C, 2 min at 95°C.
-
PCR stage 45 cycles of 3 s at 95°C, 30 s at 60°C.
-
Primer and probe info: 2019-nCoV_N1-F: ACCCCAAAATCAGCGAAAT (500nM); 2019-nCoV_N1-R: TCTGGTTACTGCCAGTTGAATCTG (500 nM); 2019-nCoV_N1-P FAM/MGB probe: ACCCCGCATTACGTTTGGTGGACC (125nM).”
Nothing spells accuracy more than a 45 cycles of PCR, isn’t it?
FINAL WORDS / PERSONAL NOTE
I have paid a HUGE personal price exposing #Pfizer/#BioNTech since Jul 21, when I published #PfizerLeak. I still do. However, in life pain is inevitable, but suffering is optional. ONLY TRUTH & LOVE CAN SAVE US FROM EVIL.