COVID-19 mRNA Shots Are Legally Not Vaccines
By Joseph Mercola
LewRockwell.com
Did you know that mRNA COVID-19 vaccines aren’t vaccines in the medical and legal definition of a vaccine? They do not prevent you from getting the infection, nor do they prevent its spread. They’re really experimental gene therapies.
I discussed this troubling fact in a recent interview with molecular biologist Judy Mikovits, Ph.D. While the Moderna and Pfizer mRNA shots are labeled as “vaccines,” and news agencies and health policy leaders call them that, the actual patents for Pfizer’s and Moderna’s injections more truthfully describe them as “gene therapy,” not vaccines.
Definition of ‘Vaccine’
According to the U.S. Centers for Disease Control and Prevention,1 a vaccine is “a product that stimulates a person’s immune system to produce immunity to a specific disease, protecting the person from that disease.” Immunity, in turn, is defined as “Protection from an infectious disease,” meaning that “If you are immune to a disease, you can be exposed to it without becoming infected.”
Neither Moderna nor Pfizer claim this to be the case for their COVID-19 “vaccines.” In fact, in their clinical trials, they specify that they will not even test for immunity.
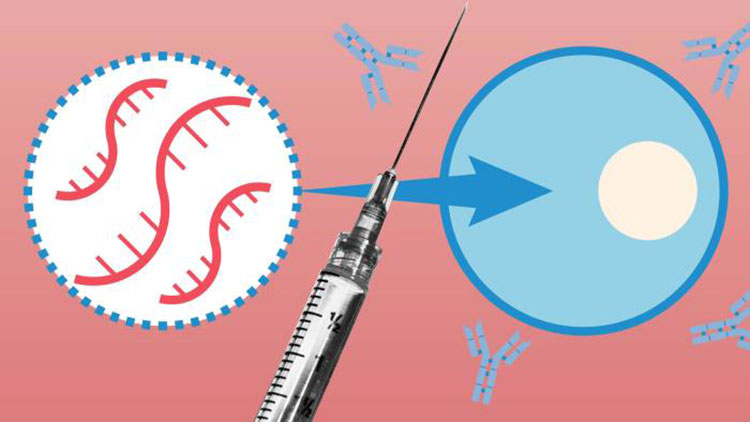
Unlike real vaccines, which use an antigen of the disease you’re trying to prevent, the COVID-19 injections contain synthetic RNA fragments encapsulated in a nanolipid carrier compound, the sole purpose of which is to lessen clinical symptoms associated with the S-1 spike protein, not the actual virus.
They do not actually impart immunity or inhibit transmissibility of the disease. In other words, they are not designed to keep you from getting sick with SARS-CoV-2; they only are supposed to lessen your infection symptoms if or when you do get infected.
As such, these products do not meet the legal or medical definition of a vaccine, and as noted by David Martin, Ph.D., in the video above, “The legal ramifications of this deception are immense.”
15 U.S. Code Section 41
As explained by Martin, 15 U.S. Code Section 41 of the Federal Trade Commission Act2 is the law that governs advertising of medical practices. This law, which dictates what you may and may not do in terms of promotion, has for many years been routinely used to shut down alternative health practitioners and companies.
“If this law can be used to shut down people of good will, who are trying to help others,” Martin says, “it certainly should be equally applied when we know deceptive medical practices are being done in the name of public health.”
Per this law, it is unlawful to advertise:
“… that a product or service can prevent, treat, or cure human disease unless you possess competent and reliable scientific evidence, including, when appropriate, well-controlled human clinical studies, substantiating that the claims are true at the time they are made.”3