Sudden Deaths Twice as High Among Vaccinated in Pfizer Trial

Sudden deaths were twice as high in the vaccine group of the original Pfizer clinical trial than in the placebo group, researchers have found, reigniting concerns about the safety of the novel mRNA drug.
The worrying finding comes from new analysis of the data from the original randomised controlled trial (RCT) of the vaccine which were released as part of legal action in Texas.
Researchers at the Health Advisory and Recovery Team (HART) found that there were four additional sudden deaths in the vaccine group than in the placebo group, all of which occurred after the first 60 days. Many assurances of vaccine safety assume that any harms will be seen within 28 days of vaccination, meaning signals after 60 days would be missed.
In all there were 12 sudden deaths with no underlying cause: eight in the vaccine group and four in the placebo group. Three occurred in each group during the first 60 days; subsequently, five occurred in the vaccine group but just one in the placebo group.
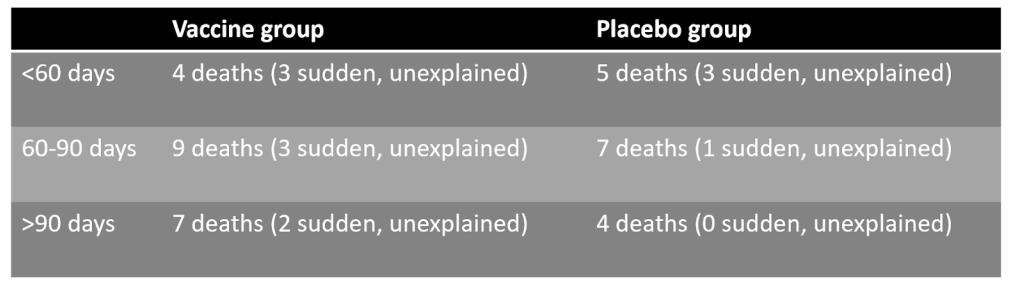
Even more concerning, say the researchers, is that these four additional sudden deaths in the vaccine group account for the whole difference in the total number of deaths between the two groups: 20 in the vaccine group versus 16 in the placebo group.
By contrast, the six reported Covid deaths in the placebo group did not result in an excess of non-sudden deaths in the placebo group, as there were 12 non-sudden deaths in each group.
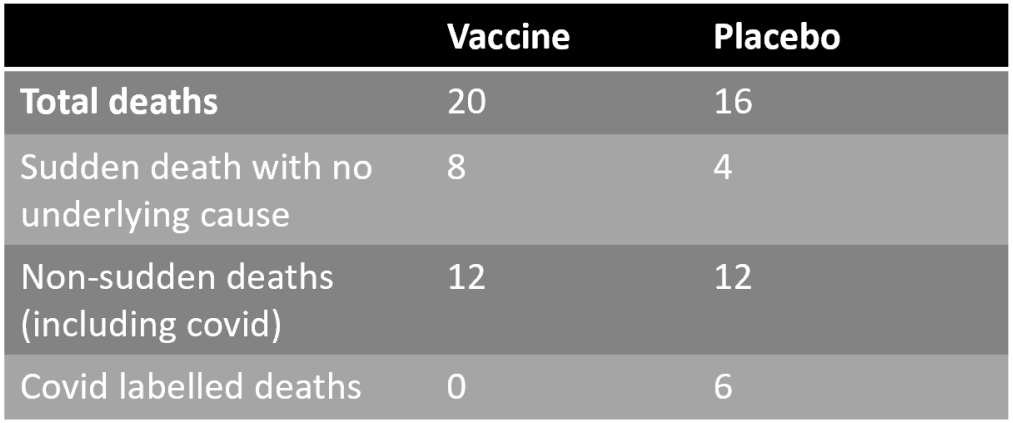
This suggests that while the vaccine did not prevent non-sudden deaths from causes such as Covid, it did cause sudden deaths.
However, while these figures from the trial are clearly a red flag that warrant urgent further investigation, the relatively small numbers involved mean the results are not statistically significant. Statistically speaking, we can’t be more than 95% confident they did not occur by chance. This is despite the trial involving 44,000 people.
If this rate of death occurred across the whole population it would equate to a vaccine mortality rate of around one per 5,000 people, which for the U.K. would mean 10,000 deaths. Such a signal must not be ignored, even if it falls below the significance threshold in this trial. What it signals is a need for further investigation to confirm or rule out the risk.
The researchers note that the six Covid deaths in the placebo group amount to 38% of the 16 total deaths in that group – an extraordinarily high proportion that calls into question how the Covid deaths were identified. Even in the U.K.’s first wave only 26% of deaths were attributed to Covid. Perhaps this is why Pfizer has said it did not rely on these Covid death figures for estimating efficacy.
The HART researchers also found evidence of potential bias in what was supposed to be a blinded trial, as the average length of time taken to report deaths in the vaccine group was two to three times longer than in the placebo group. Tellingly, perhaps, the longest delays in the reporting of vaccine-group deaths occurred ahead of the critical interim report on which the vaccine’s emergency approval was based. In that period, vaccine-group deaths took an incredible 18 days on average to report, while placebo-group deaths in the same period took just five days. Mysteriously, vaccine-group death reporting then spend up to seven days on average, once the key interim data deadline had passed.
The HART team also draw attention to dubious classification decisions, such as the 65-year-old man from Texas whose death 11 days after receiving a Covid vaccine was categorised as unvaccinated because, after unblinding, he had received the Moderna vaccine. Since many of these apparent biases favour the vaccine, any estimate of harm should be understood as a lower bound.
Sudden deaths being twice as high in the vaccine group than the placebo group of a clinical trial should have rung alarm bells among regulators right from the start. That it didn’t only adds to the wider scandal around these novel, genetic vaccines.





















